Essential Transparency
In an industry where trust is often fragile, Essential Elements is committed to building absolute assurance by going far beyond basic disclosure. We recognize the common Transparency Trap—the risk of overwhelming you with information that lacks verifiable proof—and counter it with a commitment to meticulous, multi-stage validation for every product we sell. Our process ensures your guaranteed safety and quality: we confirm potency using internal Certificate of Analysis (COA) and verify uncompromising purity through an unbiased third-party lab. We empower you with these facts: the full, batch-specific results for your exact supplement are always easily accessible online, ensuring you receive a pure and potent product every single time.

Download your
product analysis report
HERE
How to interpret test results.
This resource provides key quality control data and definitions referenced from the Indian Pharmacopoeia (IP), Ninth Edition, 2022 and ICH Q3 D. These standards ensure the safety and quality of drug substances and excipients used in India, particularly regarding chemical and elemental contaminants.
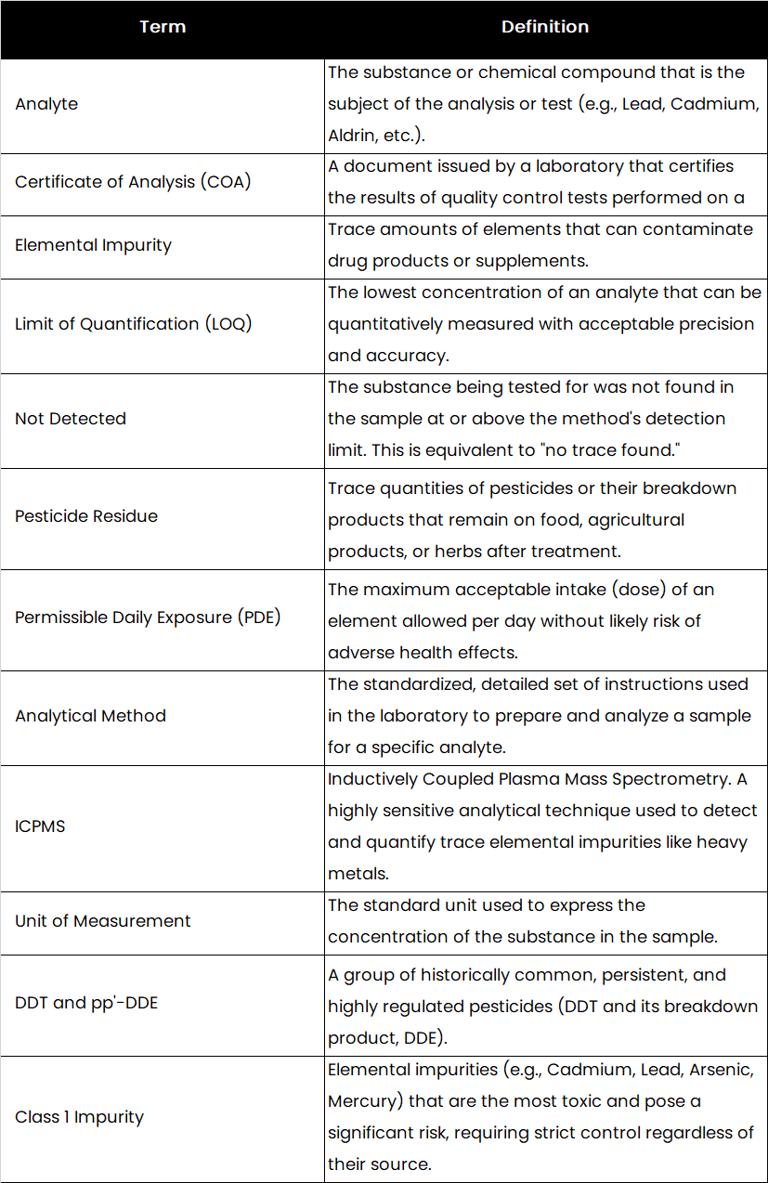
Glossary of Essential Quality Control Terms
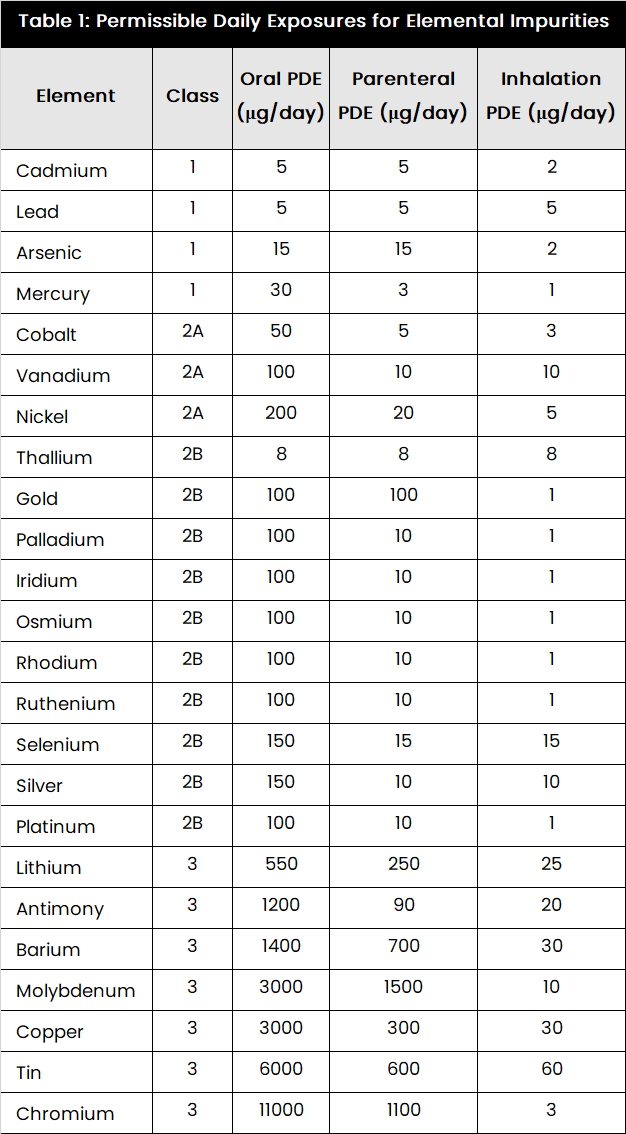
Elemental Impurity Limits (ICH Q3D/IP 2022)
Present the Permissible Daily Exposure (PDE) data clearly, acknowledging the route of administration, as this is the primary determinant for the limit. Reference the specific IP chapter/ICH Q3.
Pesticide Residue Limits
Use the table format for the pesticide limits, highlighting the general limits that apply unless a specific monograph (for a raw material) specifies otherwise.
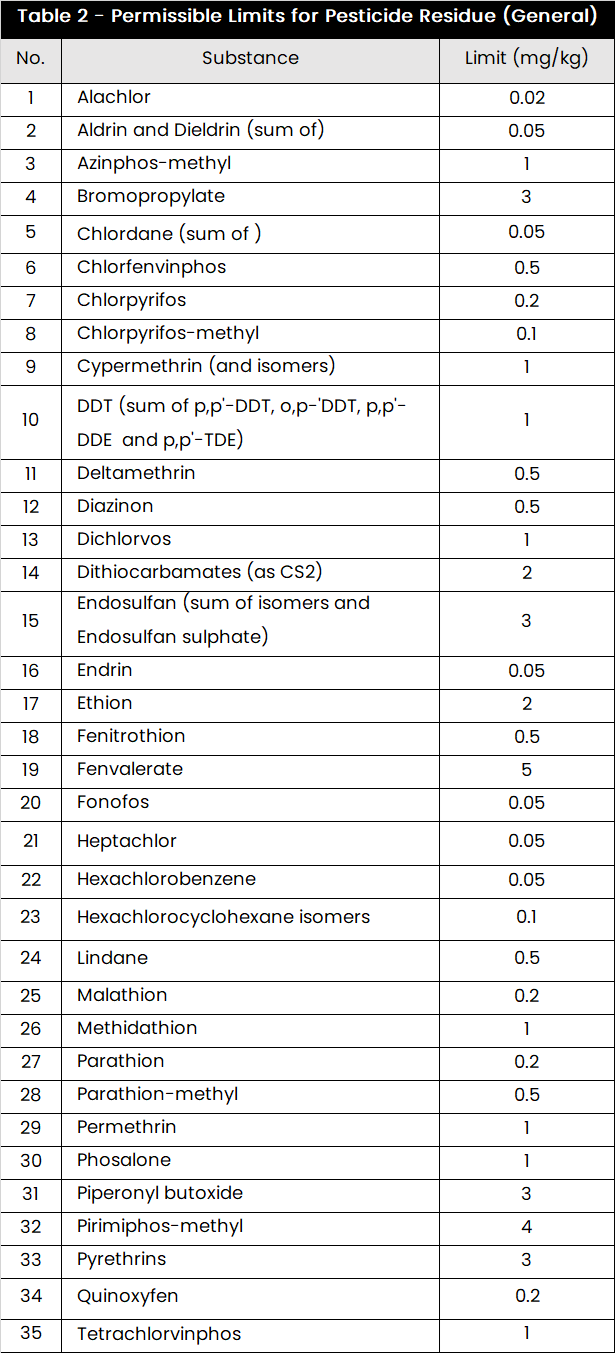
Source Reference
1) Indian Pharmacopoeia Commission (IPC). Indian Pharmacopoeia 2022 (Ninth Edition). Ghaziabad, India: IPC, Ministry of Health and Family Welfare, Government of India.
2) International Council for Harmonization (ICH). Guideline for Elemental Impurities (ICH Q3D(R1)). Geneva, Switzerland: ICH Secretariat; 2019.
USEFUL LINKS
INFORMATION
Disclaimer: * This products are not intended to diagnose, treat, cure, or prevent any diseases. They are formulated for general wellness / support only
